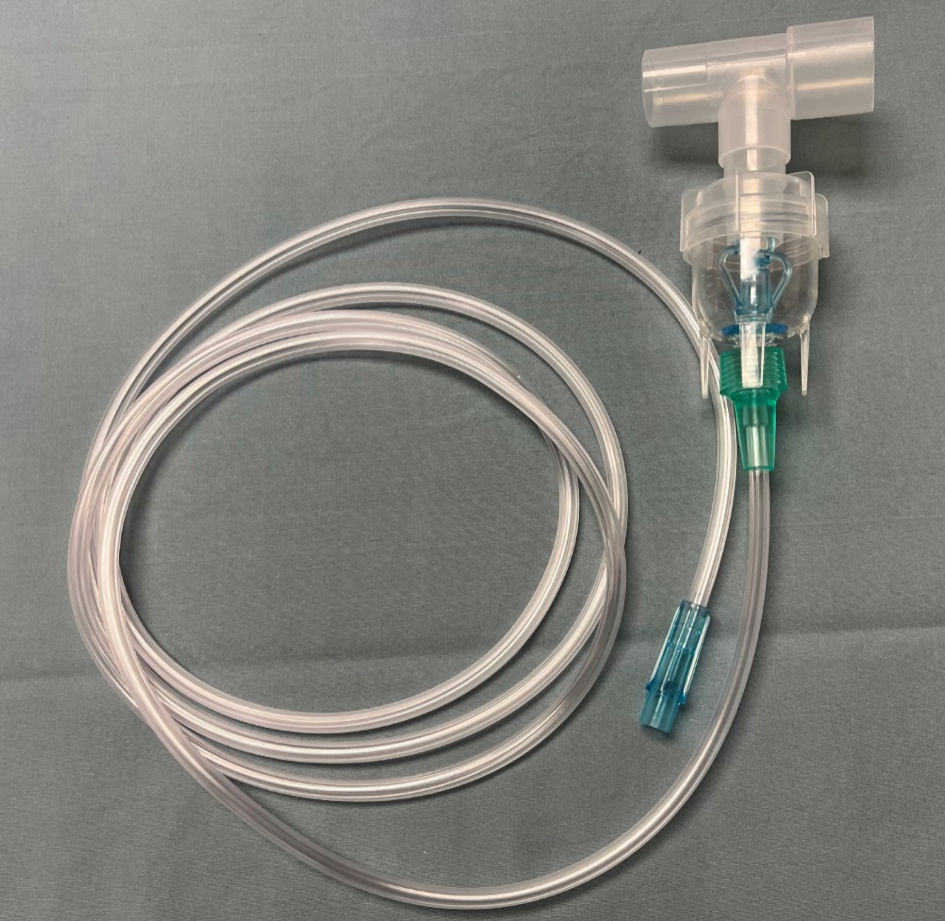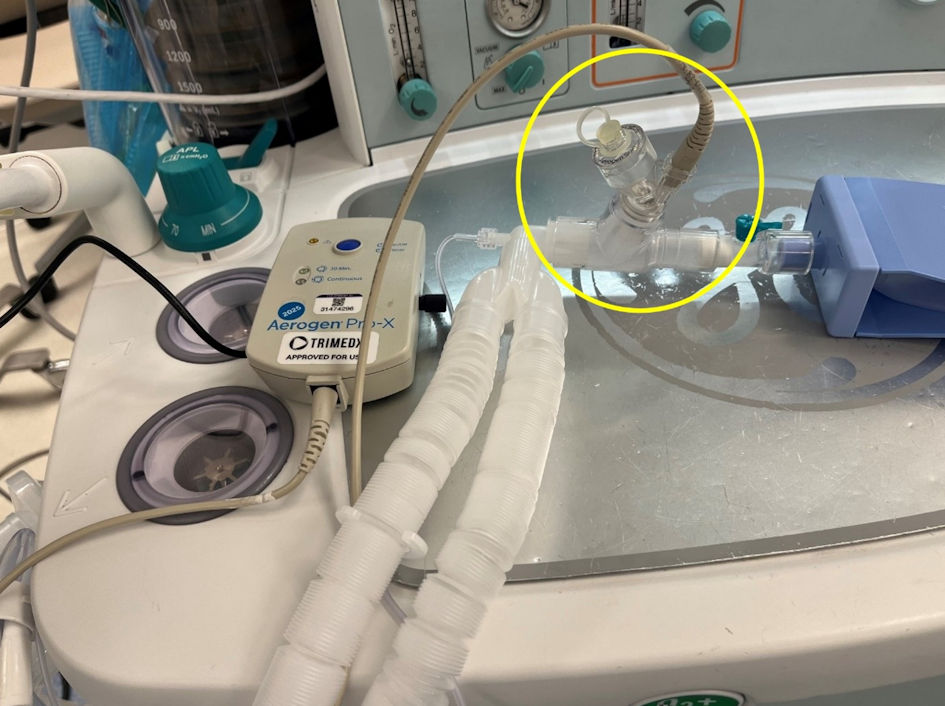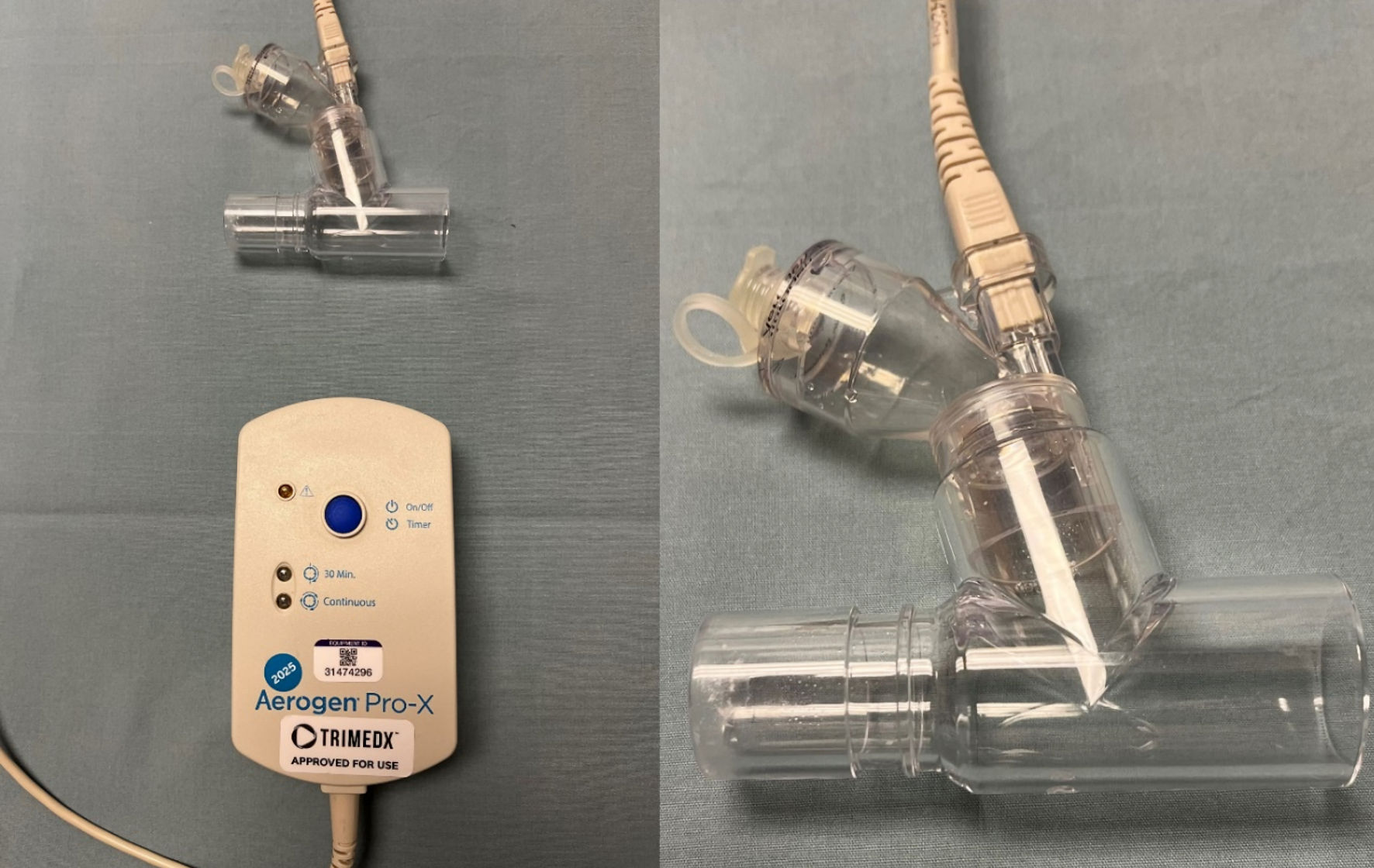
Figure 1. The Aerogen device used for nebulization during the current study. The device uses vibrating mesh technology which does not require the addition of a high-flow oxygen source which distinguishes it from commonly used high-flow nebulizing devices.