| Journal of Current Surgery, ISSN 1927-1298 print, 1927-1301 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Curr Surg and Elmer Press Inc |
| Journal website https://jcs.elmerpub.com |
Case Report
Volume 15, Number 1, March 2025, pages 26-28
Post-Pancreaticoduodenectomy Jejunal Perforation and Pancreatic Fistula Caused by Pancreatic Intraductal Stent
Heng Lva, b, Jian Shun Gec, Jie Zhanga, d
aDepartment of Gastrointestinal Surgery, The Affiliated Hospital of Yangzhou University, Yangzhou University, Yangzhou, China
bMedical College of Yangzhou University, Yangzhou, China
cDepartment of Vascular Surgery, The Affiliated Hospital of Yangzhou University, Yangzhou University, Yangzhou, China
dCorresponding Author: Zhang Jie, Department of Gastrointestinal Surgery, The Affiliated Hospital of Yangzhou University, Yangzhou University, Yangzhou, China
Manuscript submitted September 6, 2024, accepted September 23, 2024, published online February 6, 2025
Short title: Post-PD Jejunal Perforation and POPF
doi: https://doi.org/10.14740/jcs486
| Abstract | ▴Top |
Postoperative pancreatic fistula (POPF) is a highly dangerous complication following pancreaticoduodenectomy (PD). In this case, a pancreatic intraductal stent was inserted into the pancreatic duct after PD, and the pancreatic intraductal stent punctured the intestinal wall, resulting in POPF. Pancreatic fistula and digestive tract perforation for this reason have not been reported clinically and have certain significance. We present a case of a male patient who underwent PD for a malignant pancreatic tumor. He was admitted for treatment due to recurrent hematemesis and melena postoperatively. Abdominal imaging and biochemical and cytological examinations of ascitic fluid suggest that the patient may have POPF and gastrointestinal perforation. After a series of treatments, the patient’s pancreatic fistula and abdominal infection did not improve. After consultation with the patient’s family, they decided to refuse further treatment and the patient was discharged. Traditional pancreatic intraductal stents may not necessarily reduce postoperative complications and may also lead to potential adverse reactions.
Keywords: Pancreaticoduodenectomy; Postoperative complications; Pancreatic fistula; Stent
| Introduction | ▴Top |
Pancreaticoduodenectomy (PD), more commonly known as the Whipple procedure, is the gold standard surgical technique for tumors located in the head and uncinate process of the pancreas. It is the only potentially curative method available. Due to the intricate anatomy surrounding the pancreas and the involvement of multiple organ resection and anastomosis, PD is considered one of the most challenging surgeries in the field.
Fortunately, with advancements in surgical expertise and technology, the postoperative mortality rate for PD has significantly decreased over time. In the 1970s and 1980s, the postoperative mortality rate of PD was approximately 25-30%. Currently, the mortality rate after PD is only 2-4% [1]. Despite this positive trend, the occurrence and severity of postoperative complications of PD remain a concern.
Various complications may arise following PD, including surgical site infections, delayed gastric emptying, bile leakage, bleeding, and the most perilous of all, pancreatic fistula [2]. Over the past few decades, the medical community has actively explored methods to reduce the risk of postoperative pancreatic fistula (POPF). These methods include the use of different anastomotic techniques and the application of pancreatic ductal stents in PD.
In this article, we present a case of a patient with pancreatic mucinous adenocarcinoma who underwent pylorus-preserving PD. Unfortunately, the patient developed a pancreatic fistula and gastrointestinal perforation after PD. These complications were attributed to the use of pancreatic intraductal stent.
| Case Report | ▴Top |
We present a case of a male patient with pancreatic mucinous adenocarcinoma who underwent pylorus-preserving PD. After PD, the patient was repeatedly admitted to the hospital due to hematemesis, melena, and fatigue. After a series of examinations and analyses, it is believed that pancreatic fistula and gastrointestinal perforation were caused by the use of pancreatic ductal stents after PD.
Computed tomography (CT) scan revealed characteristic changes following PD, including alterations after bile-jejunum and gastro-jejunum anastomosis. High-density anastomotic shadow was observed in the local position, and the wall of gastric remnant, intestinal and gastro-jejunum anastomosis showed edema and thickened slightly. The intrahepatic and extrahepatic bile ducts were slightly dilated, and pneumobilia was observed. Furthermore, the surrounding fat space of the abdominal and pelvic cavities was blurred, especially the mesentery. The right gastric, hepatic hilar, mesenteric and paraaortic lymph nodes were enlarged with unclear boundaries. Fluid density images were observed in the edges of liver and spleen, the mesenteric space, bilateral paracolic sulci, and pelvic cavity. The intraductal stent was located in the main pancreatic duct, and the right end of the intraductal stent extended outside the intestinal lumen.
Cytological examination of ascitic fluid (on February 17, 2020) showed yellow color, positive levamisole test, white blood cell count of 9,300 × 106/L, and white blood cell differential counts of monocytes 0.1 and polymorphonuclear cells 0.9. Biochemical examination of ascitic fluid (on February 18, 2020) showed total protein of 19.6 g/L, albumin of 12.7 g/L, globulin of 6.9 g/L, lactate dehydrogenase of 2,112.7 U/L, glucose of 1.12 mmol/L, adenosine deaminase of 35.1 U/L, and high-sensitivity C-reactive protein of 54.81 mg/L. Amylase in ascitic fluid (on February 17, 2020) was 1,920.00 U/L. Bacterial culture of ascitic fluid (on February 17, 2020) showed Enterococcus faecalis. The above ascitic fluid examination showed that the patient had digestive tract perforation and pancreatic fistula after PD. Imaging examinations further revealed that the right end of the drainage tube was correctly positioned within the intestinal cavity.
After a series of treatments, the patient’s pancreatic fistula and abdominal infection did not improve. After consultation with the patient’s family, they decided to refuse further treatment and the patient was discharged.
Figures 1 and 2 show the patient’s abdominal CT images before and after surgery.
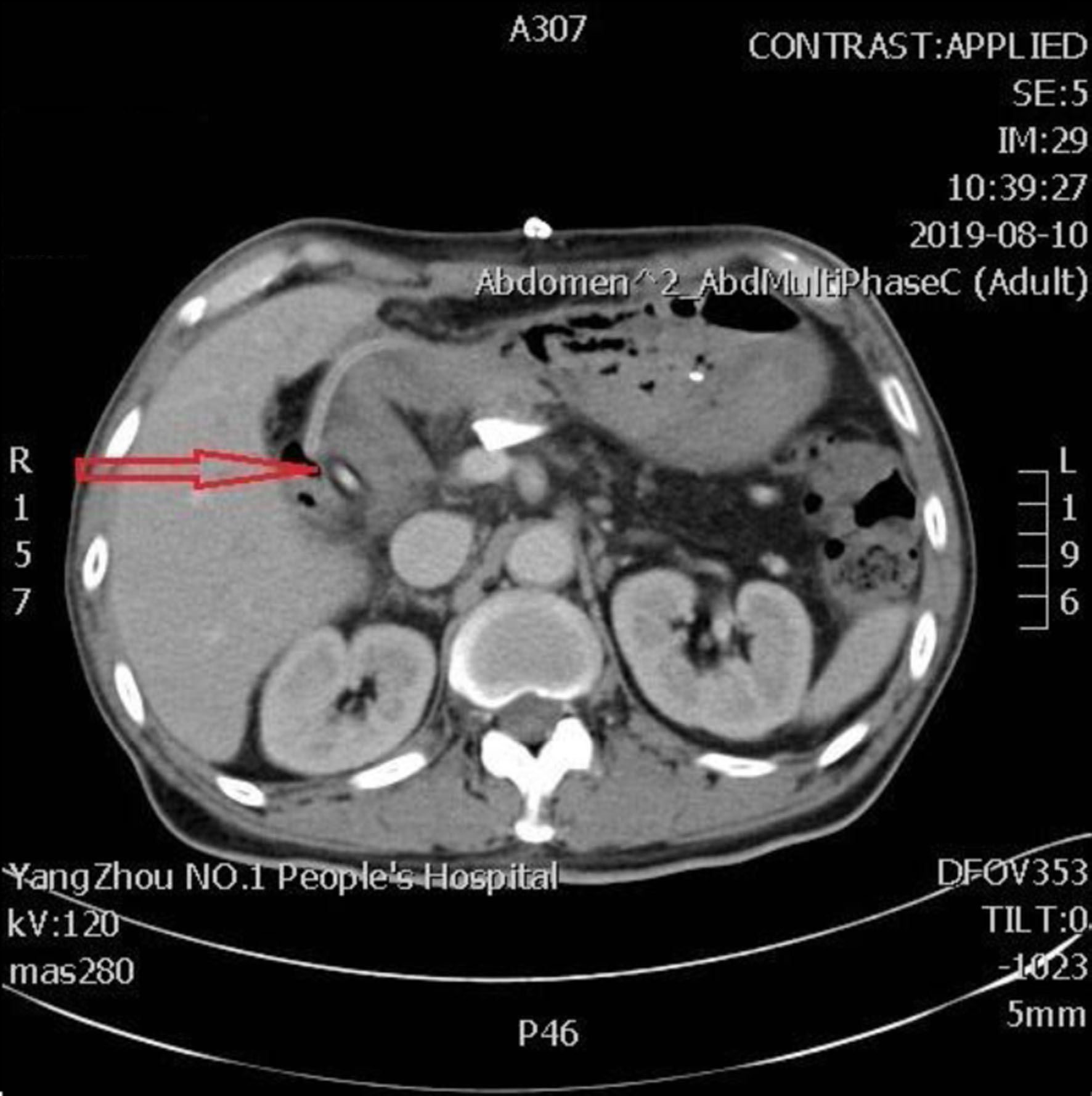 Click for large image | Figure 1. The second day after PD, the abdominal CT scan shows the pancreatic intraductal stent in place, extending from the main pancreatic duct into the intestinal lumen (arrow). CT: computed tomography; PD: pancreaticoduodenectomy. |
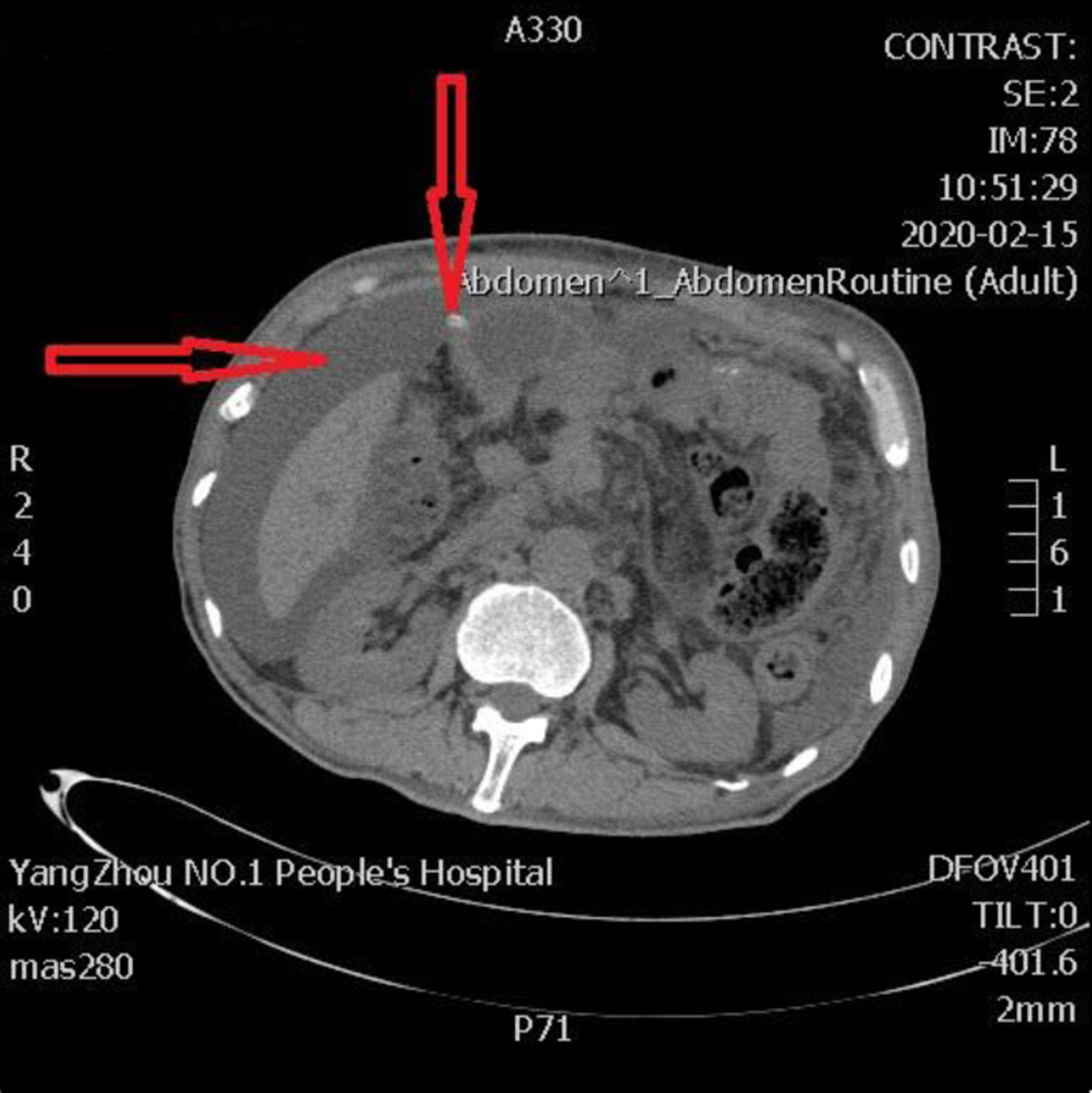 Click for large image | Figure 2. The terminal portion of the pancreatic intraductal stent breaches the intestinal wall, protruding into the extraluminal space, resulting in the formation of jejunal perforation and pancreatic fistula. The left arrow shows peritoneal effusion and the right arrow shows the perforation site. |
| Discussion | ▴Top |
In this case, the patient experienced postoperative jejunal perforation and pancreatic fistula after PD, which was caused by the use of pancreatic intraductal stent. The use of pancreatic intraductal stent to guide pancreatic fluid drainage has been a subject of study in order to reduce the occurrence of POPF. However, research findings in this area are controversial. One study comparing stent placement and non-stent placement in 444 patients undergoing PD after proximal pancreatectomy showed that the stent group had a higher rate of clinically relevant POPF (29%) compared to the non-stent group (11%) [3]. Another retrospective study of 553 patients who underwent PD found similar rates of clinically relevant POPF between the stent and non-stent groups [4]. These studies suggest that the use of pancreatic intraductal stent does not reduce the frequency or severity of POPF after pancreatic resection.
Furthermore, a study evaluating the long-term complications of PD with or without a stent in patients after PD found no significant differences between the two groups after at least 3 years of follow-up [5]. Another prospective study using magnetic resonance imaging (MRI) to evaluate the prevention of non-fibrotic pancreatic fistula after PD with an external stent showed no significant difference in the occurrence of pancreatic fistula between the stent and non-stent groups [6]. These findings suggest that the placement of intraductal stents in the pancreatic duct after pancreatic resection may not affect the occurrence of PODF and may even have negative effects.
Regarding drainage methods after PD, internal drainage is considered superior to external drainage in routine postoperative care. A prospective randomized trial showed that both internal and external drainages were safe measures for PD, but internal drainage simplified postoperative management and potentially reduced the length of hospital stay [7]. Another multicenter trial compared external stent placement to internal stent placement and found a higher incidence of clinically relevant POPF in the external stent group [8].
The pancreatic intraductal stent was placed in the main pancreatic duct and extended into the jejunal lumen, aiming to drain pancreatic fluid into the jejunum. However, in this case, the end of the intraductal stent breaks through the intestinal, leading to a pancreatic fistula and gastrointestinal perforation. The use of pancreatic intraductal stent was mentioned in the case, and the patient’s multiple hospitalizations may be due to poor postoperative healing of the anastomosis and anastomotic bleeding. The use of pancreatic intraductal stent may have contributed to the occurrence of pancreatic fistula, which worsened the patient’s condition.
Conclusions
In conclusion, this case highlights the potential complications that can arise after pancreatic resection surgery, particularly those related to anastomotic and gastrointestinal issues. Collaborative interdisciplinary management is crucial to improving treatment outcomes and prognosis in these patients. The choice of pancreatic stents and drainage methods may also play a significant role in patient outcomes. Further research is needed to determine the optimal strategies, considering individual patient factors and intraoperative conditions, in order to reduce the incidence of POPF and minimize complications.
Acknowledgments
None to declare.
Financial Disclosure
No funding was received to assist with the preparation of this manuscript.
Conflict of Interest
The authors have no competing interests that are relevant to this article’s content.
Informed Consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Author Contributions
Heng Lv, Jian Shun Ge, and Jie Zhang contributed to planning, conception and design, writing and editing various drafts of the manuscript, and read and approved the final manuscript.
Data Availability
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
| References | ▴Top |
- Narayanan S, Martin AN, Turrentine FE, Bauer TW, Adams RB, Zaydfudim VM. Mortality after pancreaticoduodenectomy: assessing early and late causes of patient death. J Surg Res. 2018;231:304-308.
doi pubmed pmc - Simon R. Complications after pancreaticoduodenectomy. Surg Clin North Am. 2021;101(5):865-874.
doi pubmed - Sachs TE, Pratt WB, Kent TS, Callery MP, Vollmer CM, Jr. The pancreaticojejunal anastomotic stent: friend or foe? Surgery. 2013;153(5):651-662.
doi pubmed - Moriya T, Clark CJ, Kirihara Y, Kendrick ML, Reid Lombardo KM, Que FG, Farnell MB. Stenting and the rate of pancreatic fistula following pancreaticoduodenectomy. Arch Surg. 2012;147(1):35-40.
doi pubmed - Suzuki S, Kaji S, Koike N, Harada N, Hayashi T, Suzuki M, Imaizumi T, et al. Pancreaticoduodenectomies with a duct-to-mucosa pancreaticojejunostomy anastomosis with and without a stenting tube showed no differences in long-term follow-up. J Hepatobiliary Pancreat Sci. 2011;18(2):258-262.
doi pubmed - Kuroki T, Tajima Y, Kitasato A, Adachi T, Kanematsu T. Stenting versus non-stenting in pancreaticojejunostomy: a prospective study limited to a normal pancreas without fibrosis sorted by using dynamic MRI. Pancreas. 2011;40(1):25-29.
doi pubmed - Tani M, Kawai M, Hirono S, Ina S, Miyazawa M, Shimizu A, Yamaue H. A prospective randomized controlled trial of internal versus external drainage with pancreaticojejunostomy for pancreaticoduodenectomy. Am J Surg. 2010;199(6):759-764.
doi pubmed - Jang JY, Chang YR, Kim SW, Choi SH, Park SJ, Lee SE, Lim CS, et al. Randomized multicentre trial comparing external and internal pancreatic stenting during pancreaticoduodenectomy. Br J Surg. 2016;103(6):668-675.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Current Surgery is published by Elmer Press Inc.